Inter-Agency Task Force (IATF) for the Management of Emerging Infectious Diseases spokesperson Cabinet Secretary Karlo Nograles on Monday revealed the general guidelines for expanded COVID-19 testing, explaining in the IATF regular briefing that expanded testing “is defined as testing all individuals who are at-risk of contracting COVID-19 infection.”
According to Nograles, “this includes the following groups: (1) suspect cases or (2) individuals with relevant history of travel and exposure (or contact), whether symptomatic or asymptomatic, and (3) health care workers with possible exposure, whether symptomatic or asymptomatic.”
Only rapid antibody-based test kits approved by the FDA and locally-validated by the RITM or the DOST may be used.
The Palace official said the sub-groups of at-risk individuals arranged in order of greatest to lowest need for testing are:
– Subgroup A: Patients or healthcare workers with severe/critical symptoms, relevant history of travel/contact
– Subgroup B: Patients or healthcare workers with mild symptoms, relevant history of travel/contact, and considered vulnerable
– Subgroup C: Patients or healthcare workers with mild symptoms, relevant history of travel/contact
– Subgroup D: Patients or healthcare workers with no symptoms but relevant history of travel/contact
Nograles stressed that all subnational laboratories in the country are directed “to allocate between 20-30% of their daily testing capacity for health workers and the remaining 70%-80% for patients.”
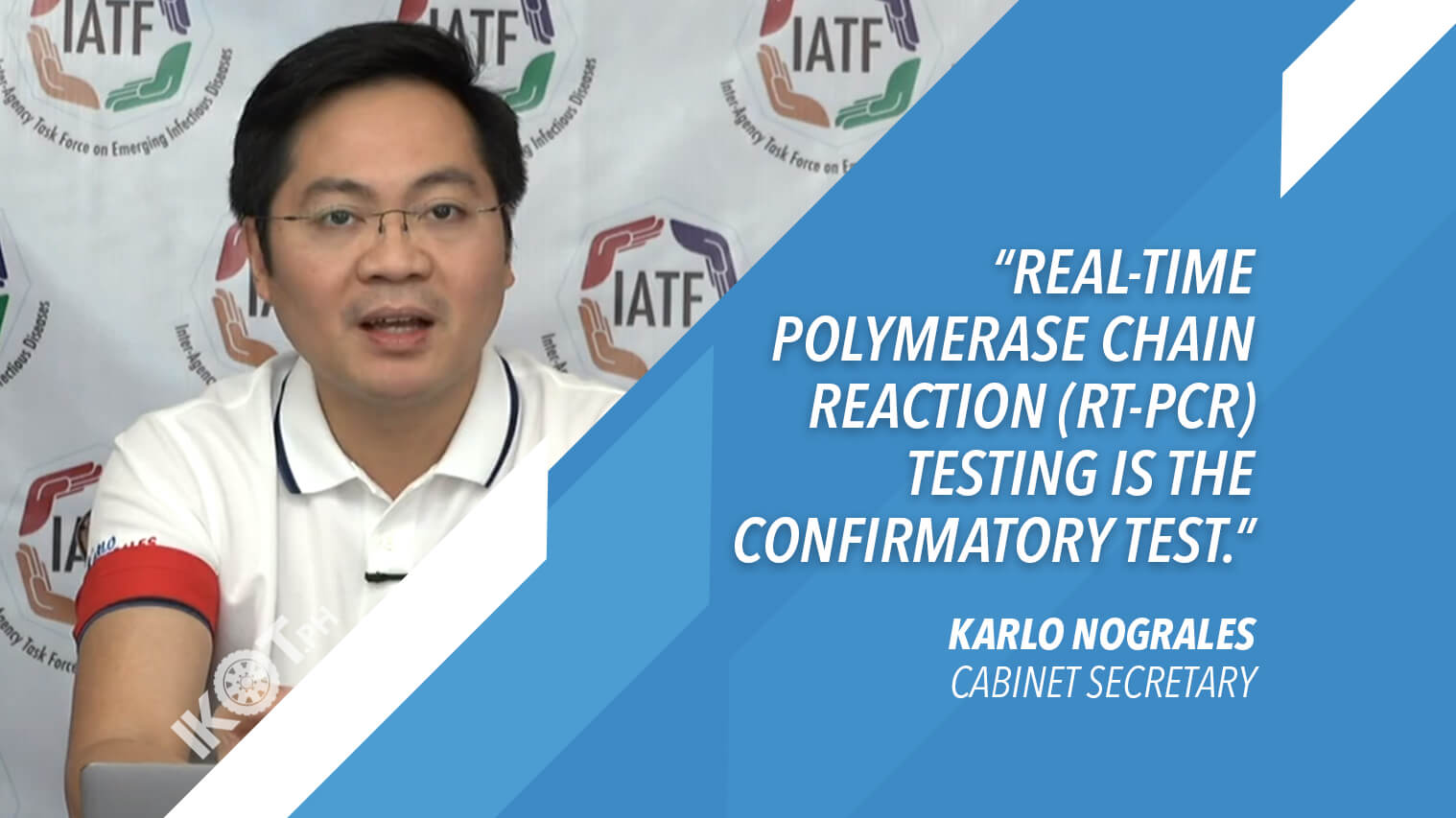
He likewise emphasized that based on current available evidence, “real-time polymerase chain reaction (RT-PCR) testing is the confirmatory test. In the Philippines, this pertains to using RT-PCR test kits that are approved by the Food and Drug Administration (FDA), and validated by the Research Institute for Tropical Medicine (RTIM).”
The Cabinet Secretary added that rapid antibody-based test kits cannot be used as standalone tests to definitively diagnose or rule out COVID-19. Nograles added that because these must be used in conjunction with RT-PCR, “care must be exercised to not unduly consume RT-PCR test kits for the sake of confirmation.”
Nograles said only rapid antibody-based test kits approved by the FDA and locally-validated by the RITM or the Department of Science and Technology (DOST) may be used.